The global halt of the Oxford-AstraZeneca phase 3 vaccine trials as the company looks into an important issue of patient safety, has given a pause to vaccine hopefuls and vaccine pushers.
It's an important, and much needed development, and one virologist we spoke with says it's 'good news'.
The Oxford-AstraZeneca candidate has been the most promising of the over 200 vaccine candidates in works, as we scramble to find a way out of the novel coronavirus pandemic.
It was the first to go into Phase 3 human trials; the phase 1/2 preliminary results have largely been promising, generating both antibody and T Cell response in participants.
In a normal scenario, the halt of an ongoing trial would have been a routine event. But this is not a normal scenario. The world is exhausted. And with politicians arm-twisting regulators to speed up the process, and announcing deadlines for the vaccines based on political events, with countries like Russia forgoing phase 3 trials to push for commercialisation, this routine event comes as a much-needed reality check.
'This is Reassuring'
A neurological symptom in one participant triggered the global hold on trials, with AstraZeneca calling the halt "an abundance of caution".
"In all large randomised efficacy trials of an investigational drug or vaccine, the drugs are given to a large heterogeneous population. Some of these people also have underlying health conditions and they may react adversely. This is exactly why we need large trials before approval," says Dr Shahid Jameel, virologist, and director, Trivedi School of Biosciences at Ashoka University.
He adds this is also not uncommon in such trials, saying, "The good thing is that there is a system in place to recognise these adverse events and address them. This is good and will build confidence in the trials."
Dr Giridhar Babu, a Bengaluru-based epidemiologist also agrees with Dr Jameel. "All ongoing vaccine trials are regulated by international standards. Every minor or major incident has to be reported to a committee, which is the Data Safety Monitoring Board. The decision to halt is with the board. This is something that is common in all vaccine trials," he says, adding,
“This is actually a relief, because it means due process is being followed and we are not rushing through the trials.”Dr Giridhar Babu, Epidimeologist
The World Health Organisation also made a very clear statement saying no large scale vaccination is likely until before mid-2021. "This phase 3 trials must take longer because we need to see how truly protective the vaccine is and we also need to see how safe it is," said Margret Harris, a WHO spokesperson.
Concerns About 1st Generation Vaccines
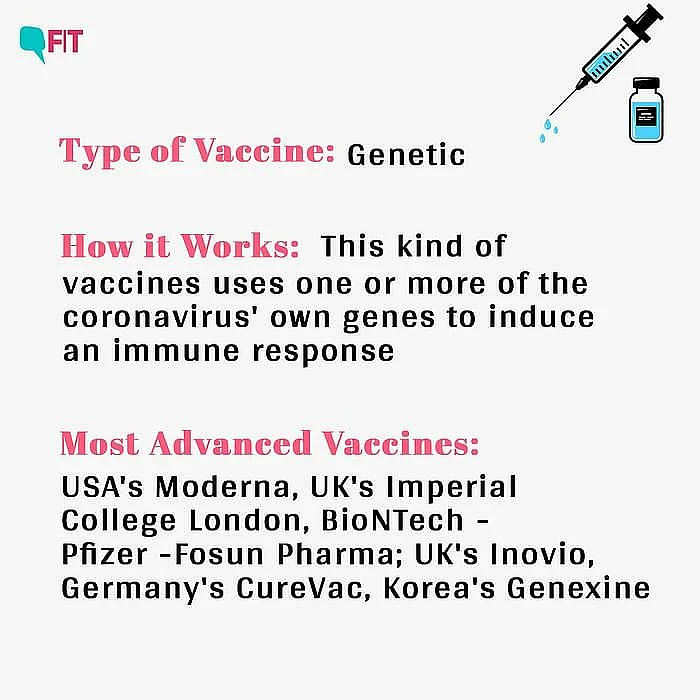
AstraZeneca's leading vaccine candidate employs a weakened version of the common cold — adenovirus that carries a gene for one of the proteins in SARS-CoV-2, the pathogen responsible for COVID-19. The adenovirus is designed to induce a protective immune system response against the coronavirus. Such a method has not been used in any approved vaccine but has been tested in experimental vaccines against other viruses, including the Ebola virus.
Public health professionals around the world have consistently raised concerns that the first-generation vaccine candidates may not be the best. FIT wrote about the concerns around rushed vaccines here.
Michael S Kinch, an expert in drug development and research at Washington University, told Washington Post, “The realistic scenario is probably going to be more like what we saw with HIV/AIDS. With HIV, we had a first generation of, looking back now, fairly mediocre drugs. I am afraid – and people don’t like to hear this, but I’m kind of constantly preaching it – we have to prepare ourselves for the idea that we do not have a very good vaccine. My guess is the first generation of vaccines may be mediocre.”
How Does a Rushed Time-Frame Affect Our Knowledge About the Vaccine?
In an earlier conversation with FIT, Anant Bhan, Adjunct Professor and Researcher in Bioethics at Mangaluru’s Yenepoya University, had explained, “The concerns lie with the key aspects of a vaccine. Some questions that need to be answered are: Do we have enough data on safety and efficacy? Do we know enough about the immune response it generates and for how long? How many doses should we administer? Is it effective across age groups?”
“When you compress the time frame, you may not be able to sufficiently answer these questions. We may not have enough knowledge of the kind and duration of the immune response a vaccine generates. If we look at data in the short term, we will only be able to understand its protective nature and efficacy for that period. Now if you only have evidence for immunity for 2-3 months and you license a vaccine for marketing, questions about its long-term protection will still remain. For that, we will have to wait for a few months, or years.”
A lot of this uncertainty emerges from the novelty of the virus. Even though we are better equipped to deal with SARS-CoV-2 than we were months ago, much of our knowledge about the virus continues to be incomplete.
In the meantime, experts are examining the 'adverse' event to see whether this was a one-off case or if there is more to the reaction. In all likelihood, if it is found that the event was not linked, vaccine trials will continue with additional monitoring.
Meanwhile, other vaccine makers in Phase 3 trials, Pfizer Inc and BioNTech, Moderna Inc, Sanofi, and China's SinoVac Biotech Ltd are other candidates in phase 3 of clinical trials.
They will all be closely monitoring the events, checking whether their vaccine candidate also produces such a response. But it's unlikely that their trials will be impacted by the AstraZeneca halt.
(The article was first published in FIT and has been republished with permission)